Announcement Details
Contact Us
Eurofins Biopharma Product Testing Denmark A/S (Eurofins BPT) provides full GMP-testing for the qualification or monitoring of your facility.
Eurofins BPT provides GMP-support for qualification /validation processes for the following contexts:
-
Clean Rooms / Controlled environments
-
Pharmaceutical and Lab water systems
-
Compressed-gas systems
-
Cleaning validation (CV)
Eurofins BPT’s specialist's offer you tailor made fully optimised solutions. We provide support for setting up protocols and /or sampling programs and identification of the risks inherent of each type of qualification / validation / monitoring.
Why Choose Eurofins BioPharma Product Testing?
-
Local support from Subject Matter Expert (SME)
-
Avoid over-doing (use our broad-vision)
-
Secure quality (integrated competences)
-
State-of-the art methods
-
On-site sampling by GMP qualified specialists
-
Transport validations with no additional costs
-
One-stop-shopping solution
-
Support during qualification and/or monitoring
-
Simplified Recovery Validation (CV)
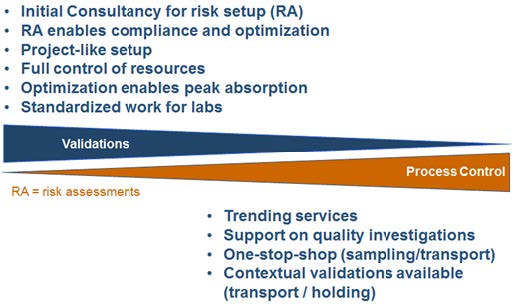
Integrated competences and optimised solutions The degree of customization of analytical methods combined with number of samples per shipment largely defines complexity of testing phase. In collaboration with expert microbiologists and chemists, we typically manage to obtain important standardization for the testing.
The main reason for the success is the use of analytical techniques which are common and well-regulated across Biopharma and Medical Device industries. Eurofins BPT avoid “re-inventing the wheel” by close collaboration with customers to commensurate sampling programs and testing with the level of risk. It is important to involve specialists in early phase of the process. By overlooking the testing plan, Eurofins BPT avoid testing-criticalities and cost-challenges. Eurofins BPT integrated competences on analytical testing, combined with a broad insight and experience- across different industries and scenarios, give a unique position to tailor your specific solutions. Eurofins BPT main scope is to always secure quality and compliance.
The approach we prefer to use
-
Define risks
-
Define protocols and sampling plans
-
Secure adherence to state-of-the-art methods
-
Support decision making processes with data